The Science Behind the PreTRM® Test
Until recently, clinicians have had limited resources for predicting the risk of spontaneous preterm birth. Traditional indicators of increased risk — such as short cervical length and a positive history for previous spontaneous preterm birth — fail to detect 80% of spontaneous preterm births.
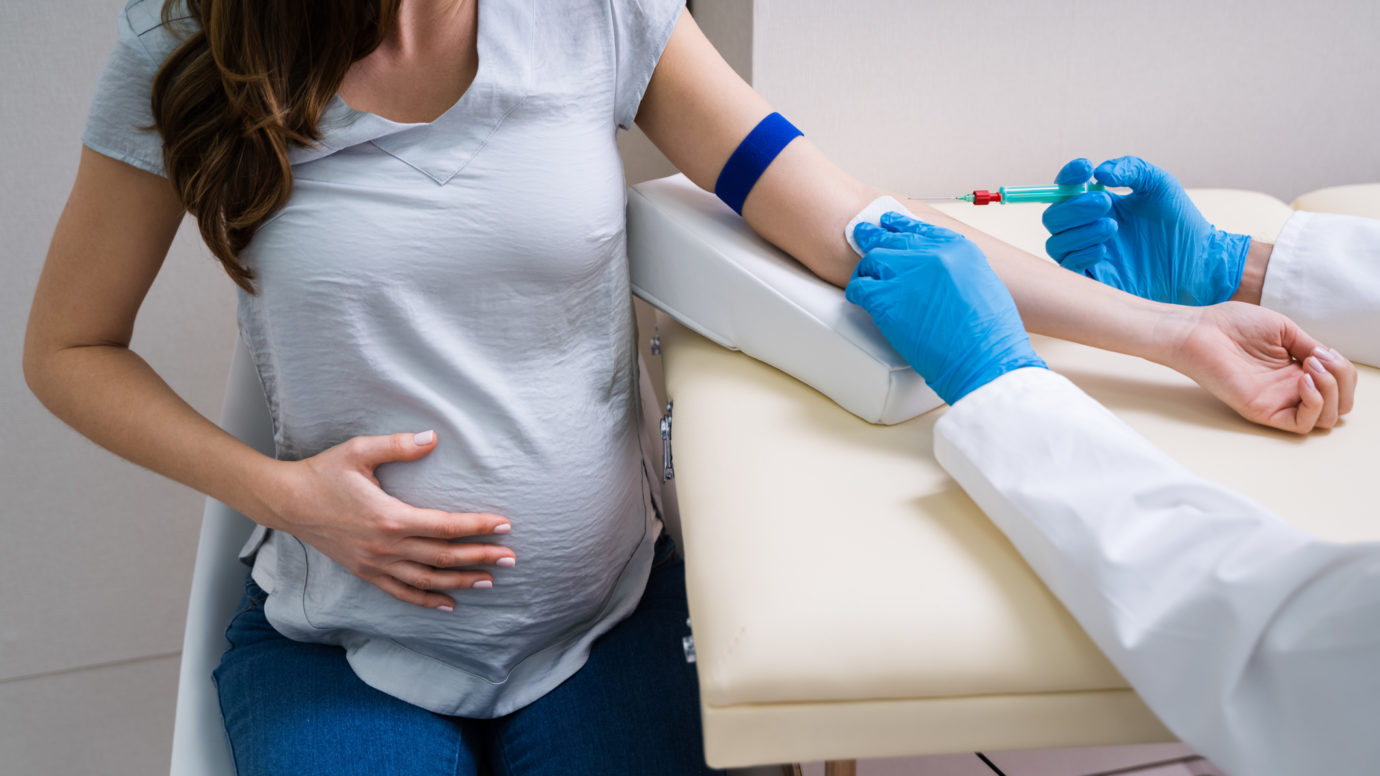

The Need for an Accurate and Reliable Risk Predictor in the Clinical Setting
Given the limitations of current risk factors to predict the risk of premature birth, scientists at Sera set out to discover biomarker prediction to develop a test that would be:
- Accurate and reliable at identifying pregnancies at increased risk of spontaneous preterm birth
- Performed early enough in pregnancy to be able to improve outcomes
- Minimally invasive
- Timed to coincide with planned obstetrical visits

The PAPR Study:
Discovery and Validation
The groundbreaking Proteomic Assessment of Preterm Risk (PAPR) study1, published as an Editor’s Choice article in American Journal of Obstetrics and Gynecology, was conducted to discover, verify, and validate protein biomarkers in the serum of pregnant women.
Based on best practices for “omics” research, PAPR enrolled 5,501 pregnant women at 11 Institutional Review Board (IRB)-approved sites across the United States. Blood draws were performed weekly at 17-28 weeks’ of gestation and samples were analyzed using proteomics, bioinformatics, and multidimensional data analysis.
Contact Us
Contact us for more information about preterm birth and the PreTRM Test.
References
- Saade GR, et al. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol. 2016 May;214(5):633.e1-633.e24.
- Sitar T, et al. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci USA. 2006 103: 13028-13033.
- Grishkovskaya I, et al. Resolution of a disordered region at the entrance of the human sex hormone-binding globulin steroid-binding site. J Mol Biol. 2002 318: 621-626.