Request the PreTRM Test
There are two ways to get the PreTRM Test.

Ordering the PreTRM Test Online is an Easy Process
- Find out if PreTRM is right for you. Enter information to meet needed criteria for testing, apply for financial assistance, and enter payment information.
- Collect: After receiving the PreTRM Blood Sample Kit, follow the instructions included to collect the blood sample between 18 and 20⁶ᐟ⁷ weeks of your pregnancy.
- Ship: Drop your kit off at any FedEx, or you can contact the Sera Customer Support to request a pick-up: (801) 990-6600.
- If your order is confirmed by an independent physician of record, we will run your test and send you a notification when the results are ready.
- Download your results to share them with your healthcare provider for interpretation.*
* Some states require that we also send your results to your treating healthcare provider.
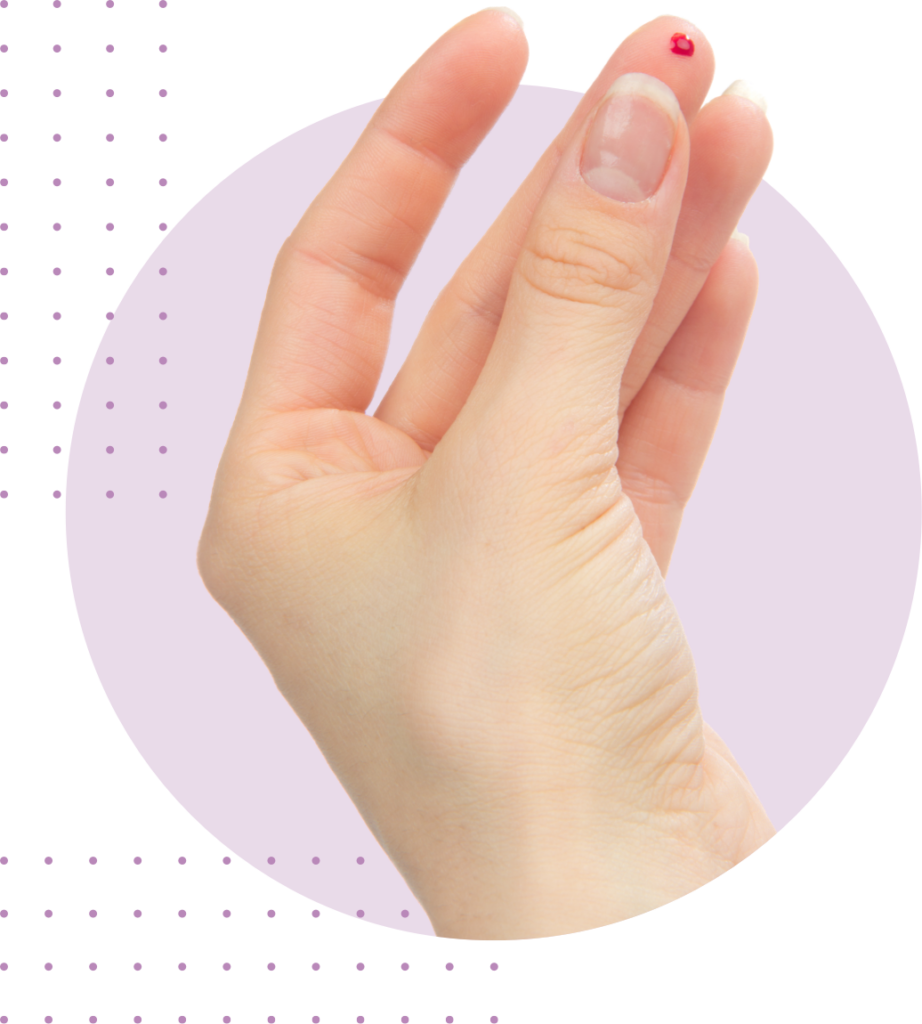
Just a quick finger prick to collect your sample
Each kit has step-by-step instructions and the contents needed to collect and ship your sample. Download the English/Spanish Instructions today.
Calculate your blood draw window for the PreTRM® Test
The PreTRM® Test is accurate at predicting your risk of a premature birth with a single blood draw during the 18th through 20th week of your pregnancy. If you request the PreTRM® Test online, make sure you have time to collect your blood sample during the window.
Based on the due date of {due_date}, the blood draw should occur between {start_date} and {end_date}
Your doctor will confirm your blood draw window, and our team’s Customer Support will help you with scheduling and reminders.
Important Test Information
The PreTRM® test is recommended for use in women 18 years or older with a singleton pregnancy to predict the risk of spontaneous preterm birth. Results should be interpreted by a healthcare provider in the context of medical history, clinical signs and symptoms. A test result of Lower Risk does not rule out spontaneous preterm birth. A test result of Higher Risk does not predict with certainty a spontaneous preterm birth.
The Sera clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. The PreTRM® test was developed and its performance characteristics were determined by Sera. The PreTRM® test has not been cleared or approved by the Food and Drug Administration. The PreTRM® clinical laboratory is regulated under CLIA to perform high-complexity testing. The PreTRM® test is intended for clinical purposes.
Questions?
Any Issues with Sample Collection or Questions? Contact PreTRM
Customer Support for questions or additional information at (801) 990-6600
or support@pretrm.com